 The
Laboratory Response Network (LRN) is the nation's laboratory emergency response system for biological, chemical and radiological threats and other public emergencies such as natural disasters. Founded by APHL, the Centers for Disease Control and Prevention and the Federal Bureau of Investigation in 1999 to improve US readiness for bioterrorism, the LRN remains a valuable resource for law enforcement and public health officials. It links local, state and federal public health laboratories with sentinel clinical, food, veterinary, environmental and agricultural laboratories; and military and international laboratory centers.
The
Laboratory Response Network (LRN) is the nation's laboratory emergency response system for biological, chemical and radiological threats and other public emergencies such as natural disasters. Founded by APHL, the Centers for Disease Control and Prevention and the Federal Bureau of Investigation in 1999 to improve US readiness for bioterrorism, the LRN remains a valuable resource for law enforcement and public health officials. It links local, state and federal public health laboratories with sentinel clinical, food, veterinary, environmental and agricultural laboratories; and military and international laboratory centers.
Over time, the Department of Defense has become an LRN stakeholder, joining in strategic planning and supporting LRN biological response activities, notably the 2014 response to the outbreak of Ebola.
APHL & the LRN
APHL supports the LRN through training, quality improvement initiatives, surge capacity and exercise planning, policy development and fostering of partnerships. The association supports the following activities:
-Plans and convenes the biennial LRN National Meeting to provide LRN stakeholders with current information on preparedness collaborations and emerging technologies, share model practices, discuss solutions to ongoing challenges, address training needs and explore the future of the LRN to stay ahead of threats
-Assists in planning the biannual LRN Conventional Methods courses, training 32 laboratorians per year in classical microbiological detection techniques
-Assists laboratories in equipment procurement for various multi-center validation studies to improve technology in the LRN
-Convenes biannual in-person meetings of the LRN Operational Workgroup and the LRN Joint Leadership Committee to discuss strategic planning and operations.
LRN-B: Tiered and Integrated for Rapid Detection, Response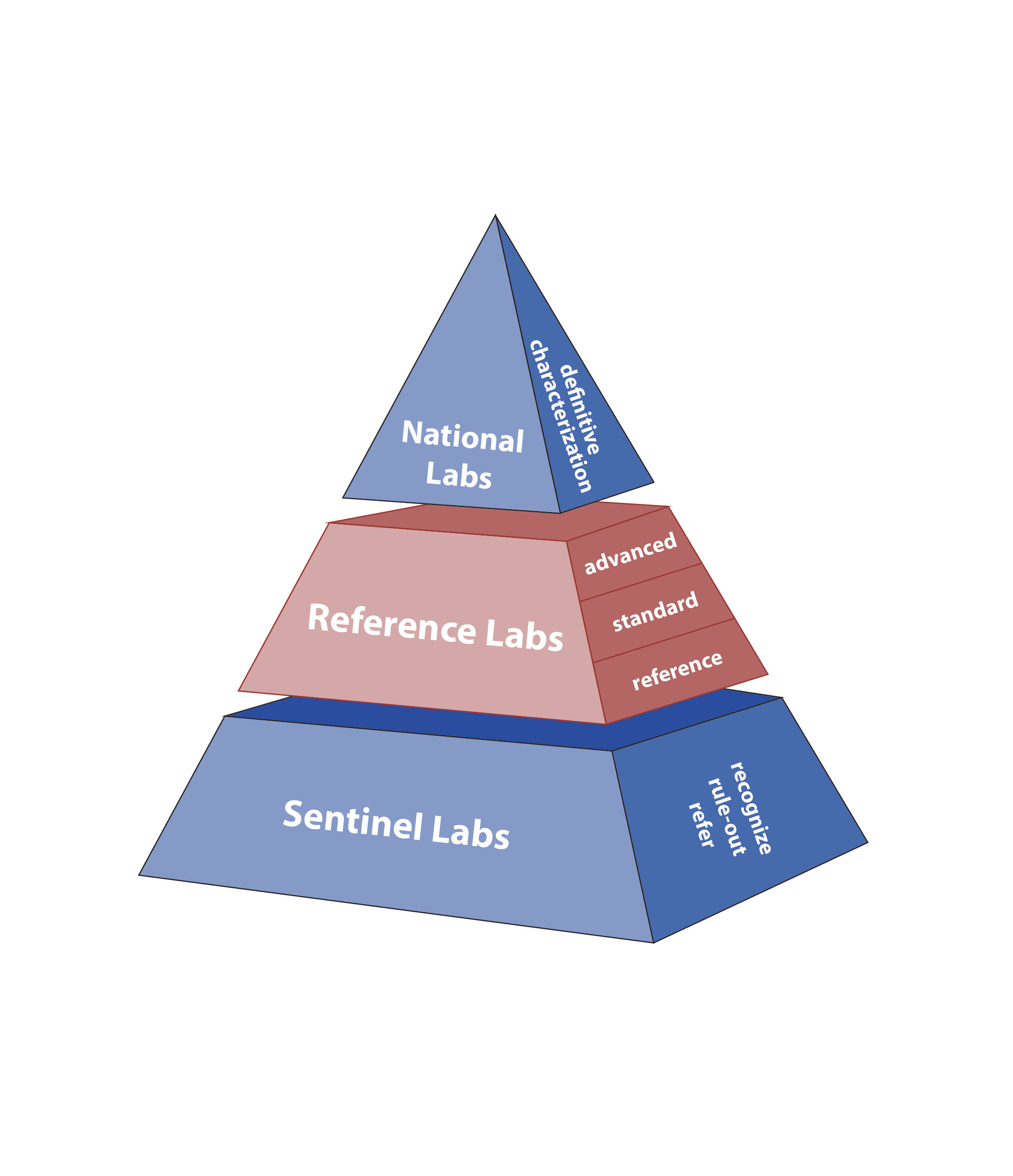
The LRN for Biological Threats Preparedness (LRN-B) is organized as a tiered pyramid of sentinel clinical, reference and national laboratories. These laboratories are integrated into a single network for rapid detection and response to threats. In 2014, the reference tier was further categorized into reference, standard and advanced laboratories based on capabilities. This reconfiguration:
- Further standardized testing capabilities
- Supported quality improvements initiatives
- Improved flexibility
- Expanded existing capabilities for biothreat agent and emerging infectious disease testing
Federal Investment in LRN
In the years following the LRN’s founding, significant federal and state investment in public health preparedness strengthened laboratory capability and capacity to respond to health threats, both man-made and naturally occurring. This investment proved invaluable in responding to Hurricanes Katrina and Sandy and other natural disasters. It also readied public health laboratories to respond to the nationwide outbreak of 2009 H1N1 influenza and to emerging diseases such as Middle East Respiratory Syndrome (MERS) and Ebola.
For more information, contact Tyler Wolford, MS, senior specialist, Laboratory Response Network at
tyler.wolford@aphl.org or
240.485.2775.